Description
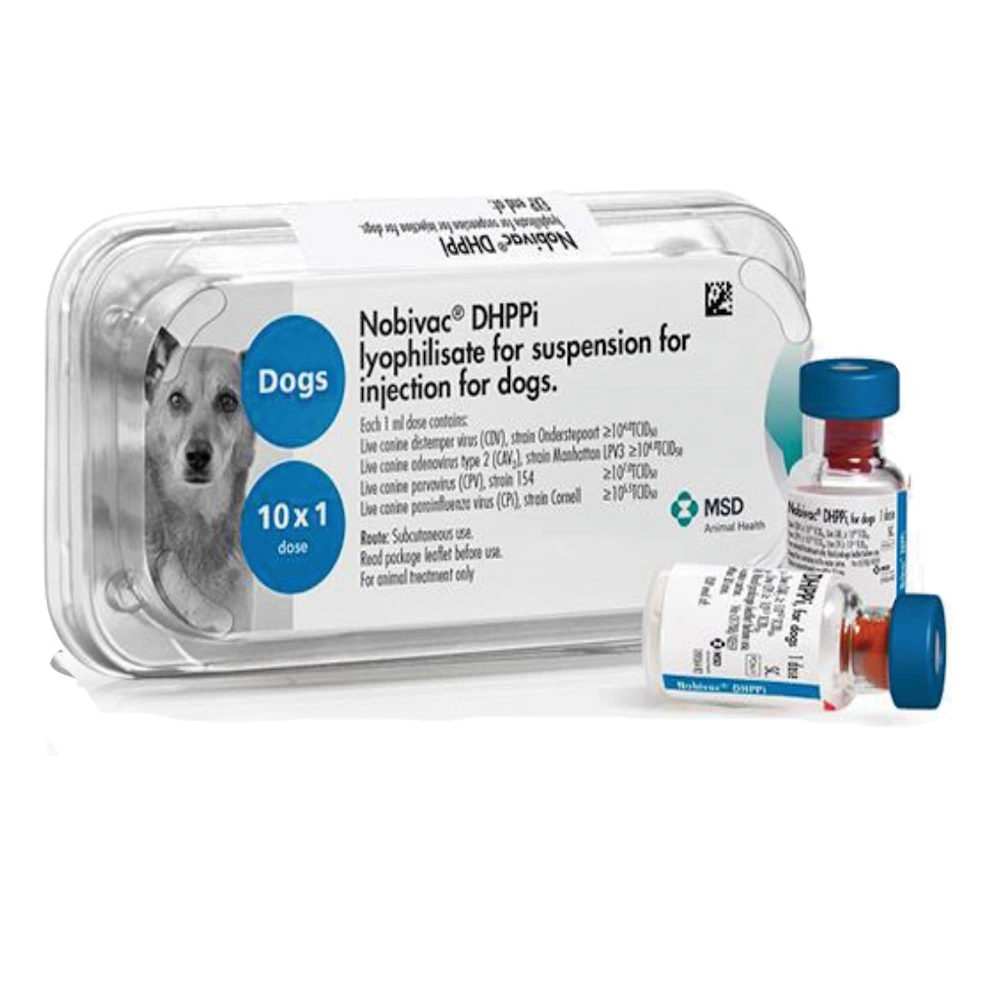
Nobivac DHPPi
Nobivac DHPPi provides immunisation to dogs for distemper, hepatitis (canine adenovirus), canine parvovirus and canine parainfluenza and is typically given in combination with a Nobivac leptospirosis vaccine (Nobivac Lepto2 or L4). It is recommended that puppies may be immunised from 6 weeks of age, 2-4 weeks apart, with a second dose from 10 weeks of age. Onset of immunity is one week after the second vaccine. In dogs over 10 weeks of age a single dose is likely to be sufficient to immunise.
Immunity provided against these core diseases is long lasting, with a minimum duration of immunity and recommended revaccination interval of 3 years. An annual health check and vaccination against canine leptospirosis is still recommended for all dogs.
Presentation
Off-white or cream-colored lyophilisate for suspension for injection
Active ingredients
Nobivac DHPPi contains live, attenuated, freeze dried canine distemper virus, canine adenovirus-2 (CAV2), canine parvovirus and canine parainfluenza virus.
Each dose of 1 ml contains:
Live canine distemper virus (CDV), strain Onderstepoort ≥ 104.0 TCID50*
Live canine adenovirus type 2 (CAV2), strain Manhattan LPV3 ≥ 104.0 TCID50*
Live canine parvovirus (CPV), strain 154 ≥ 107.0 TCID50*
Live canine parainfluenza virus (CPi), strain Cornell ≥ 105.5 TCID50*
TCID50*= Median Tissue Culture Infective Dose
Target species
Dog
Indications for use
Nobivac DHPPi is indicated for active immunisation of dogs to:
- Prevent mortality and clinical signs caused by canine distemper virus infection
- Reduce clinical signs of infectious hepatitis and viral excretion due to canine adenovirus type 1 infection
- Prevent mortality, clinical signs and viral excretion following canine parvovirus infection
- Reduce clinical signs and viral excretion caused by canine parainfluenza virus infection
- To reduce clinical signs of respiratory disease and viral excretion following adenovirus type 2 infection
Packaging
Nobivac DHPPi is presented in APET-Plastic trays containing 10 or 50 single dose vials. Nobivac Solvent is packaged separately.
Legal category
MSD Product