Description
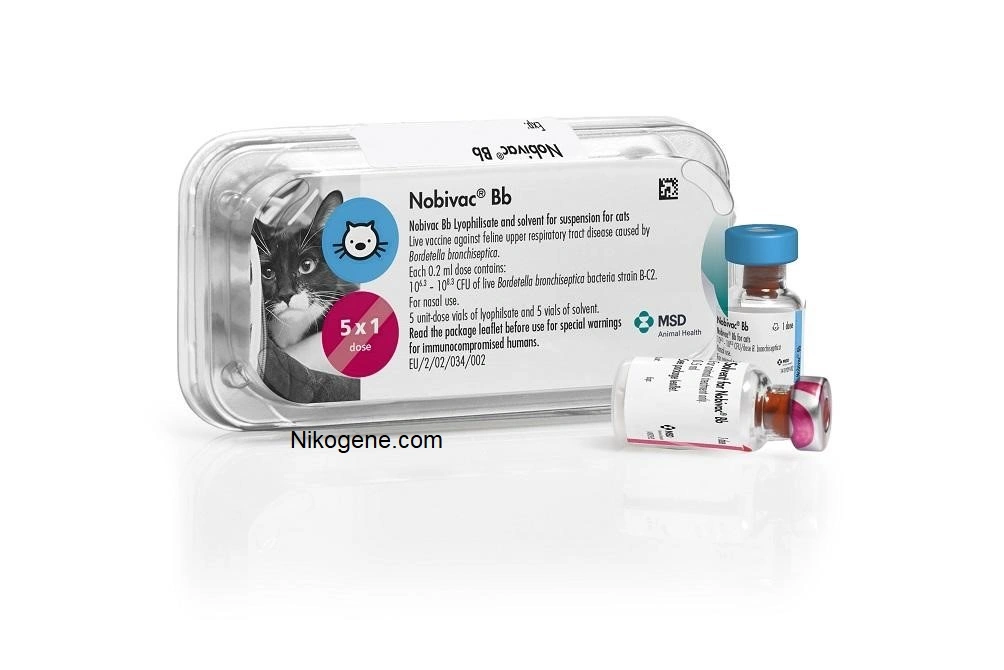
Nobivac® Bb
lyophilisate and solvent for suspension for cats
Presentation:
Lyophilisate: Off-white or cream-coloured pellet
Solvent: clear colourless solution
Active substance:
Each dose (0.2 ml) of reconstituted suspension contains:
106.3-108.3 colony forming units (CFU) of live Bordetella bronchiseptica bacteria strain B-C2
Target species:
Cats
Indications:
For active immunisation of cats, of 1 month of age or older to reduce clinical signs of Bordetella bronchiseptica associated upper respiratory tract disease.
Onset of immunity:
Onset of immunity was established in 8 week old cats as early as 72 hours after vaccination.
Duration of immunity:
The duration of immunity is up to 1 year.
No data on the influence of maternal antibodies on the effect of vaccination with Nobivac Bb for cats are available.
Contraindications:
None known.
Special warnings:
If any antibiotic is administered within one week after vaccination, the vaccination should be repeated after the antibiotic treatment has been completed.
Special precautions for use in animals:
Only healthy cats should be vaccinated.
Sneezing by cats after administration does not adversely affect the efficacy of the veterinary medicinal product.
Vaccinated animals can spread the vaccine strain of Bordetella bronchiseptica for six weeks, and there may be intermittent shedding for at least one year.
Although the risk of immunocompromised humans becoming infected with Bordetella bronchiseptica is extremely low, it is advised that cats, which are in close contact with immunocompromised humans are not vaccinated with this vaccine.
Dogs, and unvaccinated cats may react to the vaccine strain with mild and transient respiratory signs. Other animals, such as rabbits and small rodents, have not been tested.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
In case of accidental self-administration, seek medical advice immediately and show the package leaflet or the label to the physician.
Appropriate disinfection procedures should be used following use of this live bacterial vaccine.
Although the risk that immunocompromised humans become infected with Bordetella bronchiseptica is extremely low, such individuals should be aware that cats can shed the organism for up to 1 year after vaccination.
Adverse reactions:
After administration, occasionally sneezing, coughing, mild and transient discharge from the eyes or nose, may occur. In cats that show more severe signs, appropriate antibiotic treatment may be indicated.
Use during pregnancy, lactation or lay:
Do not use in pregnant or lactating queens.
Interaction:
No information is available on the safety and efficacy of this vaccine when used with any other veterinary medicinal product. A decision to use this vaccine before or after any other veterinary medicinal product therefore needs to be made on a case by case basis.
Amounts to be administered and administration route:
Nasal use.
Vaccination scheme:
One dose, of 0.2 ml of reconstituted vaccine at least 72 hours prior to period of anticipated risk.
Allow the solvent to reach room temperature. Aseptically reconstitute the lyophilisate with 0.3 ml of the sterile solvent provided. Shake well.
Withdraw 0.2 ml of reconstituted vaccine into a 1 ml or 2 ml syringe, remove the needle and administer the whole contents of the syringe into one of the cat’s nostrils.
The head of the cat should be held with its nose pointing upwards and its mouth closed, so that it is forced to breathe through its nostrils. Place the syringe in front of one of the nostrils and carefully administer the whole contents of the syringe into the nasal cavity via this nostril. The vaccine is administered directly from the tip of the syringe onto the opening of the nostril and enters the nasal cavity during inhalation.
Overdose:
No adverse reactions other than those mentioned were observed after the administration of an overdose of the vaccine.
Withdrawal period:
Not applicable.
Shelf life:
Shelf life of the veterinary medicinal product as packaged for sale: 5 years.
Shelf life after reconstitution: use within 4 hours.
Store:
at 2 – 8°C Protect from light
Presentations:
Carton box containing 5 vials of 1 dose of lyophilisate and 5 vials of solvent Plastic box containing 5 vials of 1 dose of lyophilisate and 5 vials of solvent
Not all presentations may be marketed.
MSD Product